Duchenne Muscular Dystrophy Market Size, Demands, Growth, Forecast & Report 2032 | UnivDatos
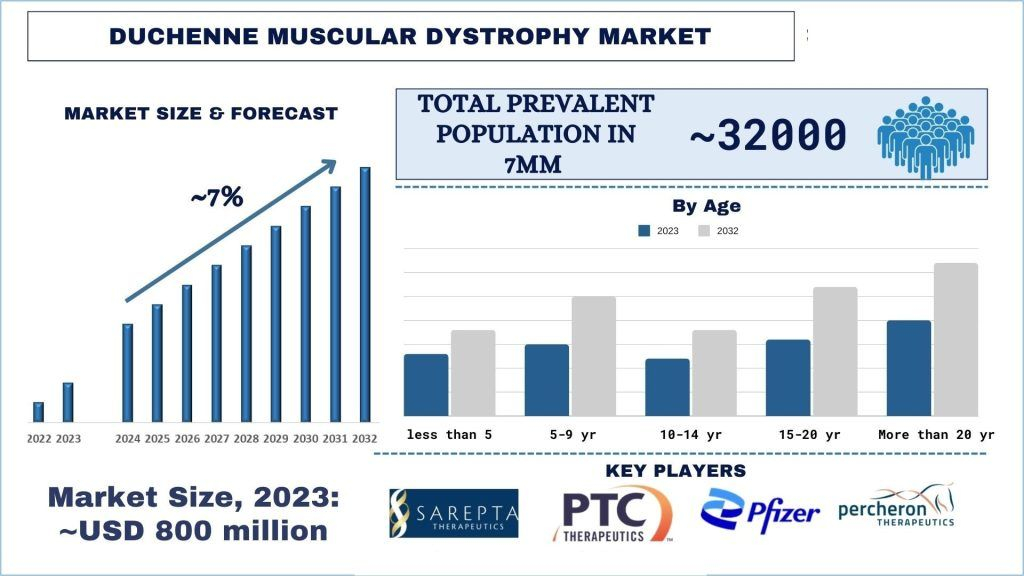
The Duchenne Muscular Dystrophy Market was valued at approximately USD 800 million in 2023 and is expected to grow at a strong CAGR of around ~7% during the forecast period (2024-2032)
Market Size and Growth Projections: The Duchenne Muscular Dystrophy (DMD) market in the 7MM is projected to grow significantly, with a compound annual growth rate (CAGR) of ~7% from 2024 to 2032. This growth is driven by the increasing prevalence of DMD and advancements in genetic therapies.
Emerging Therapeutics: The report highlights the pipeline of innovative treatments, including gene therapies and exon-skipping drugs. Notable examples include PF-06939926, Givinostat, and Vamorolone, which are expected to receive regulatory approvals and drive market growth.
Regulatory Landscape: The U.S. FDA's supportive regulatory framework, including orphan drug designations and accelerated approval pathways, has facilitated the rapid development and approval of DMD therapies. These regulatory incentives are crucial for attracting investment and expediting the availability of new treatments.
Prevalence and Diagnosis Rates: In 2023, the 7MM accounted for approximately ~30000-35000 prevalent cases of DMD. The report provides detailed epidemiological data, showing a higher diagnosis rate due to improved genetic testing and awareness, which are essential for early intervention and management.
Patient Support and Advocacy: Parent Project Muscular Dystrophy (PPMD) and the Muscular Dystrophy Association (MDA) are pivotal in supporting patients, funding research, and advocating for beneficial policies. Their efforts have significantly impacted the DMD landscape by ensuring continuous focus and resources for the disease.
This is mainly because expanding treatment options will give patients access to a broader range of treatment options, enabling growth and penetration in the Duchenne Muscular Dystrophy industry as more therapies receive regulatory approval. For instance, on March 21, 2024, the U.S. Food and Drug Administration approved Duvyzat (givinostat) oral medication for treating Duchenne Muscular Dystrophy (DMD) in patients six and older. Duvyzat is the first nonsteroidal drug approved to treat patients with all genetic variants of DMD. This expansion will enhance personalized care approaches, allowing for better disease management based on individual patient needs.
Access sample report (including graphs, charts, and figures): https://univdatos.com/reports/duchenne-muscular-dystrophy-market?popup=report-enquiry
The ability to tailor treatments to individual genetic profiles and specific disease manifestations enhances patient outcomes and drives market demand. Some of the key developments influencing the Duchenne Muscular Dystrophy market include:
On March 28, 2024, Critical Path Institute’s (C-Path) Duchenne Regulatory Science Consortium (D-RSC) announced the launch of a groundbreaking model-based Clinical Trial Simulator (CTS) specifically designed to improve the design of efficacy studies for potential therapies for Duchenne muscular dystrophy (DMD). This pioneering Drug Development Tool is set to positively impact the medical research community by significantly optimizing clinical trial design.
On January 5, 2023, Sarepta Therapeutics announced the signing of a commercial supply agreement for Catalent to manufacture delandistrogene moxeparvovec (SRP-9001), Sarepta’s most advanced gene therapy candidate for the treatment of Duchenne muscular dystrophy (DMD).
On September 30, 2022, Solid Biosciences Inc., a life sciences company focused on advancing meaningful therapies for Duchenne muscular dystrophy (Duchenne), announced the closing of its acquisition of AavantiBio, Inc., a privately held gene therapy company focused on transforming the lives of patients with Friedreich’s ataxia and rare cardiomyopathies. The acquisition included its pipeline assets and net cash.
Click here to view the Report Description & TOC: https://univdatos.com/reports/duchenne-muscular-dystrophy-market
The regulatory framework's focus on personalized medicine has led to the approval of treatments tailored to individual needs and desired outcomes, enhancing patient satisfaction and driving market demand. Some of the most approved treatments for Duchenne Muscular Dystrophy include:
The treatment of Duchenne muscular dystrophy has included significant non-steroidal advances that offer new hope for managing the disease. Among these, Duvyzat (givinostat) stands out as a noteworthy development. Approved by the FDA in March 2024, Duvyzat is a non-steroidal histone deacetylase (HDAC) inhibitor that has demonstrated efficacy in reducing muscle deterioration across all genetic forms of Duchenne muscular dystrophy.
On June 22, 2023, Sarepta Therapeutics announced U.S. Food and Drug Administration (FDA) accelerated approval of ELEVIDYS, an adeno-associated virus-based gene therapy for the treatment of ambulatory pediatric patients aged 4 through 5 years with Duchenne muscular dystrophy (DMD) with a confirmed mutation in the DMD gene.
Conclusion
In conclusion, the Duchenne Muscular Dystrophy (DMD) market is poised for substantial growth, fueled by increasing prevalence, innovative therapies, and a supportive regulatory landscape. With a projected CAGR of ~7% from 2024 to 2032, the market registered USD 800 million in 2023. The market is witnessing significant advancements, such as the approval of Duvyzat and ELEVIDYS, marking a shift towards personalized medicine. Moreover, initiatives like the launch of the Clinical Trial Simulator and strategic agreements between companies further indicate a promising future for DMD treatment development and patient care.
Key Offerings of the Report
Epidemiology, Market Size, Trends, & Forecast by Revenue | 2024−2032.
Market Dynamics – Leading Trends, Growth Drivers, Restraints, and Investment Opportunities
Market Segmentation – A detailed analysis by Marketed Therapies, Emerging Therapies, and 7MM Region
Competitive Landscape – Top Key Vendors and Other Prominent Vendors
Contact Us:
UnivDatos
Email: contact@univdatos.com
Contact no: +1 978 7330253
Website: www.univdatos.com
Linked In: https://www.linkedin.com/company/univ-datos/
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Oyunlar
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness