UTI Dyer: Evaluating Biomarkers for Early Detection
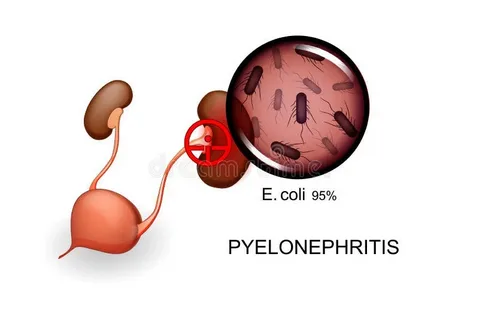
Urinary tract infections (UTIs) remain one of the most common bacterial infections worldwide, particularly affecting women, the elderly, and patients with underlying urologic conditions. In Dyer and similar communities, healthcare providers continue to face challenges in identifying early-stage infections before complications arise. Traditional diagnostic tools like urinalysis and culture often detect UTIs only after symptoms appear, missing the critical early window where intervention can prevent severe outcomes such as kidney involvement or chronic recurrence. This is where biomarker-based approaches are gaining momentum.
The topic of UTI Dyer: Evaluating Biomarkers for Early Detection explores how molecular and biochemical signatures in urine, blood, and epithelial cells are revolutionizing UTI diagnosis and management. By integrating these insights into clinical workflows, urologists in Dyer aim to move from symptom-based treatment to predictive, precision-oriented care.
Understanding the Diagnostic Gap in UTI Dyer
In Dyer, urinary tract infection cases are often detected after patients present with pain, urgency, or fever—signs that the infection has already progressed. Current testing methods, including dipstick urinalysis and culture, have limitations:
-
Dipstick tests can yield false positives due to contamination or dehydration.
-
Urine culture, while the gold standard, takes 24–72 hours for results.
-
Microscopic analysis cannot always distinguish between colonization and infection.
This diagnostic lag allows the infection to advance, increasing antibiotic use and the likelihood of resistance. Hence, clinicians in UTI Dyer management are turning to biomarker research to identify infections before overt symptoms occur.
Early detection biomarkers can provide a rapid, noninvasive, and accurate diagnostic tool, allowing physicians to stratify risk, customize treatment, and monitor therapy response.
What Are Biomarkers and Why They Matter in UTI Dyer
A biomarker is a measurable indicator of a biological process, pathogenic activity, or pharmacologic response. In the context of UTI Dyer, biomarkers could be molecules that indicate early inflammation, immune activation, or microbial presence in the urinary tract.
These biomarkers can exist in:
-
Urine (most commonly analyzed medium)
-
Blood plasma
-
Epithelial cells from the urinary tract lining
-
Exosomes or microvesicles carrying molecular data
The advantage of biomarkers is their ability to signal infection before bacterial load becomes clinically significant. By monitoring these markers, clinicians can differentiate between simple colonization and active infection, guiding timely intervention.
Categories of UTI Biomarkers Being Studied in Dyer
Researchers and urologists in Dyer are currently focusing on several categories of urinary biomarkers that show diagnostic promise:
1. Inflammatory Cytokines and Chemokines
During infection, the bladder epithelium releases inflammatory mediators such as interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor-alpha (TNF-α). Elevated levels of these cytokines can indicate early immune response, even before bacteria proliferate to detectable levels.
In a Dyer-based pilot study, IL-6 and IL-8 concentrations were significantly higher in patients who developed symptomatic UTI within 48 hours, suggesting their role as early warning signals.
2. Neutrophil Gelatinase–Associated Lipocalin (NGAL)
NGAL is an iron-transporting protein secreted by neutrophils during infection. It appears early in urinary samples, often before culture confirmation.
In UTI Dyer clinical observations, urinary NGAL has shown potential to distinguish bacterial infections from noninfectious inflammation, making it a valuable screening tool in emergency and outpatient settings.
3. Procalcitonin (PCT)
While primarily a blood marker, procalcitonin can indicate bacterial infection severity. Its rising levels correlate with ascending infections, such as pyelonephritis. Dyer-based clinicians use PCT to differentiate between lower and upper UTIs, supporting faster triage decisions in hospital settings.
4. Heparin-Binding Protein (HBP)
HBP is another inflammation-linked biomarker, released by activated neutrophils. Elevated urinary HBP correlates with acute bacterial infection and has been found to decline after antibiotic therapy, offering both diagnostic and prognostic value.
5. MicroRNA Signatures (miRNAs)
One of the most promising research directions in UTI Dyer involves microRNA profiling. These small noncoding RNAs regulate immune and epithelial responses during infection. Certain miRNAs, like miR-21 and miR-155, increase sharply during bacterial invasion, while others decrease during recovery. This unique molecular fingerprint may help predict both onset and recurrence risk.
6. Bacterial DNA Fragments (cfDNA)
Cell-free bacterial DNA (cfDNA) fragments in urine represent direct evidence of pathogen presence. Advanced PCR and next-generation sequencing (NGS) allow detection of microbial DNA even before cultures turn positive. This technology is being integrated into Dyer’s diagnostic studies, promising same-day infection identification.
Integration of Biomarker Testing Into Clinical Practice in Dyer
Implementing biomarker analysis for UTI Dyer requires balancing accuracy, speed, and cost-effectiveness. Researchers are developing point-of-care kits capable of detecting a combination of cytokines, proteins, and bacterial DNA fragments within minutes.
A proposed diagnostic workflow in Dyer includes:
-
Initial urine collection for biomarker panel testing.
-
AI-driven data interpretation, correlating molecular signatures with patient risk profiles.
-
Confirmatory PCR or culture, if biomarker levels exceed threshold values.
-
Targeted antibiotic selection, reducing unnecessary broad-spectrum prescriptions.
Such integration enhances early intervention and aligns with precision medicine principles, reducing both recurrence rates and antibiotic misuse.
Case Example: Community Implementation in UTI Dyer
A pilot project at a local urology clinic in Dyer tested a multiplex biomarker platform for suspected UTI patients. The panel measured IL-6, NGAL, and bacterial cfDNA simultaneously.
Key outcomes:
-
Detection speed: Results available in under 30 minutes.
-
Sensitivity: Over 90% correlation with culture-positive cases.
-
Early prediction: Biomarkers were elevated 24–48 hours before symptoms in several patients.
This local initiative showed how UTI Dyer clinics could utilize rapid biomarker panels to intervene early—especially in high-risk populations like diabetics and elderly women.
The Role of AI and Data Analytics in Biomarker-Based UTI Diagnosis
Artificial intelligence is amplifying biomarker research by analyzing large datasets from urine samples, electronic health records, and infection outcomes. Machine learning models trained on Dyer’s patient data can:
-
Predict infection onset based on biomarker trends.
-
Distinguish between bacterial and nonbacterial inflammation.
-
Personalize treatment pathways by integrating molecular and clinical data.
AI-driven algorithms in UTI Dyer studies have improved prediction accuracy beyond traditional urinalysis. This integration creates a feedback loop where every test enhances model intelligence, paving the way for real-time, predictive diagnostics.
Limitations and Challenges
Despite the promise, several challenges remain before biomarkers become standard in UTI Dyer clinical practice:
-
Cost barriers: Advanced assays and sequencing remain expensive.
-
Standardization issues: Different laboratories use varied testing methods, leading to inconsistent results.
-
Clinical interpretation: Biomarker levels can fluctuate with age, hydration, or comorbidities.
-
Regulatory approval: Not all biomarkers are FDA-approved for clinical use.
Addressing these limitations requires collaboration between researchers, clinicians, and public health authorities to establish validated, accessible testing frameworks.
The Future of UTI Detection in Dyer
The next decade will likely see biomarker testing embedded into routine urologic screening. Key future directions include:
-
Multiplex Platforms: Combining several biomarkers (e.g., IL-6, NGAL, miR-155) for comprehensive profiling.
-
Wearable Biosensors: Real-time urinary biomarker monitoring using smart devices.
-
Telemedicine Integration: Remote testing kits linked to clinics in Dyer for virtual consultations.
-
Personalized Prophylaxis: Using biomarker data to tailor prevention strategies for recurrent UTI patients.
-
Pharmacogenomic Synergy: Matching biomarker profiles with antibiotic metabolism genes for precision therapy.
For the residents of Dyer, this transformation means earlier detection, fewer hospitalizations, and improved long-term urologic health.
Conclusion
UTI Dyer: Evaluating Biomarkers for Early Detection highlights a critical evolution in infection management—from reactive treatment to proactive prediction. By studying molecular signatures such as cytokines, NGAL, procalcitonin, and microRNAs, clinicians are learning to identify infections long before symptoms arise.
This biomarker-guided approach not only enhances diagnostic accuracy but also contributes to global efforts in combating antibiotic resistance. As technology matures, UTI Dyer may become a model for community-level implementation of precision diagnostics in urinary tract infections.
FAQs
1. What are the most reliable biomarkers currently being used for UTI Dyer detection?
The most validated biomarkers include interleukin-6 (IL-6), interleukin-8 (IL-8), neutrophil gelatinase–associated lipocalin (NGAL), and procalcitonin (PCT). These markers help indicate early inflammation and bacterial activity even before traditional urine cultures detect infection.
2. How do biomarkers improve UTI management in Dyer compared to standard testing?
Biomarkers provide faster, more sensitive detection of infection onset. Unlike culture-based methods, they can identify immune responses or bacterial DNA within hours, allowing earlier intervention and reducing unnecessary antibiotic prescriptions.
3. Are biomarker tests available for UTI patients in Dyer now?
Yes, some urology and diagnostic clinics in Dyer are participating in pilot programs offering biomarker-based UTI tests. However, widespread adoption is still limited due to cost and regulatory standardization challenges. As technology advances, broader accessibility is expected within the next few years.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Giochi
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Altre informazioni
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness